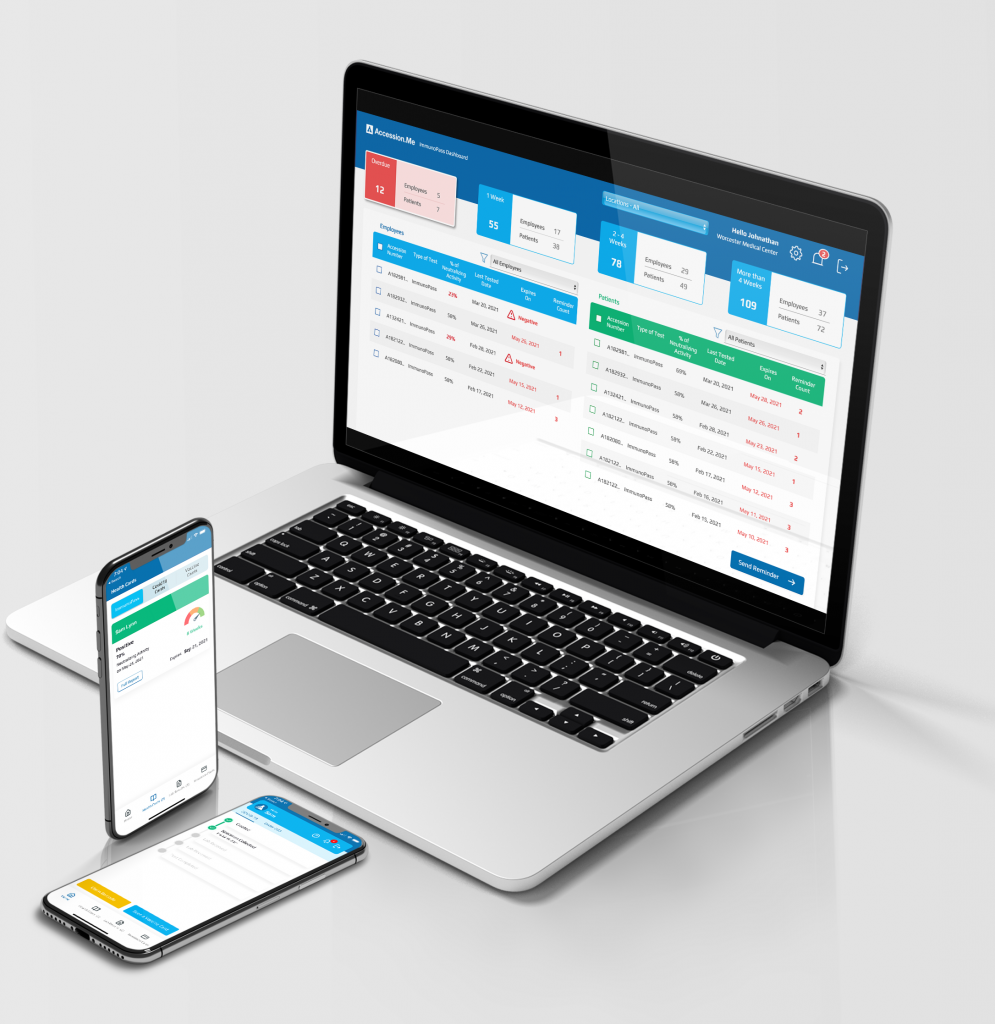
A NEW COMPREHENSIVE IMMUNITY ASSAY FOR SARS-CoV-2*
Neutralizing Antibodies SARS-CoV-2
- Percentage*
- Percentage*
- Prior COVID-19 infection
- No Prior COVID-19 infection
EVE Patient
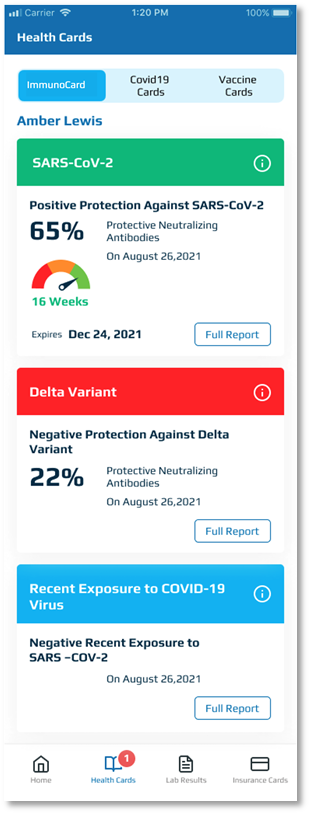
A NEW IMMUNOCARD™
Percentage protection for
- SARS-CoV-2*
- Delta Variant*
Recent exposure to COVID-19**
Notification to retest
Access to full laboratory report
Information screen
The ImmunoCard™ provides the user with a summary of a panel of tests performed looking at neutralizing antibody (NAb) levels against SARS-CoV-2, the Delta variant, and recent exposure to SARS-CoV-2*
Neutralizing antibodies (NAb) are the only antibodies produced by the body’s immune system that will prevent the SARS-CoV-2 virus from penetrating and infecting the cell
MORE INFORMATION
*The neutralizing antibody test has been authorized by the FDA under an Emergency Use Authorization (EUA). This test is only authorized for the duration of the declaration that circumstances exist for justifying the authorization of emergency use in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564 (b) (1) of the Act, 21 U.S.C. 360bbb-3 (b) (1), unless the authorization is terminated or revoked sooner. This test has been authorized only for detecting the presence of antibodies against SARS-CoV-2, not for any other viruses or pathogens.
**The semi-quantitative and Delta neutralizing antibody components of the test have been modified under CLIA guidelines to report total blocking activity percentages (%), quantification of blocking antibodies in IU/ml, anti-Delta variant neutralizing antibody blocking activity percentages (%), automation, and finger stick specimens.
**This N1 fragment antibody test was developed, and its performance characteristics determined, by Healix Pathology, LLP laboratory (CLIA# 06D2174124). It has not been cleared or approved by the FDA. The laboratory is regulated under CLIA and is qualified to perform high-complexity testing. This test is used for clinical purposes. It should not be regarded as investigational or for research.
This testing was performed in the Healix Pathology, LLP laboratory (CLIA# 06D2174124), located at 4800 Fournace Place, Suite BW3, Bellaire, TX 77401.
Decisions based on these test results should only be made after consultation with your physician.
If you have any questions on the test please contact Healix Pathology at: (281) 846 6723